is volumetric pipette dilution|volumetric pipet procedure : mail order We also can accomplish the same dilution in two steps using a 50-mL pipet and 100-mL volumetric flask for the first dilution, and a 10-mL pipet and a 50-mL volumetric flask for the second dilution. The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the volumetric .
We strive to play a crucial role in furthering the advancement of aerated, lightweight cementitious technologies here in the US and around the world, as a legacy building material, for .
{plog:ftitle_list}
AAC is a concrete-based material used for both exterior and interior construction. One of its advantages is quick and easy installation because the material can be routed, . See more
A volumetric pipet should not be "blown out" to eject all liquid at the tip because volumetric pipets are calibrated in a manner that takes into account the solution which remains at the tip due to surface tension.Glass transfer pipets are more precise than measuring (Mohr) pipets. Volumetric flasks are more accurate and precise than Erlenmeyer flasks, graduated cylinders and beakers. The volume of .
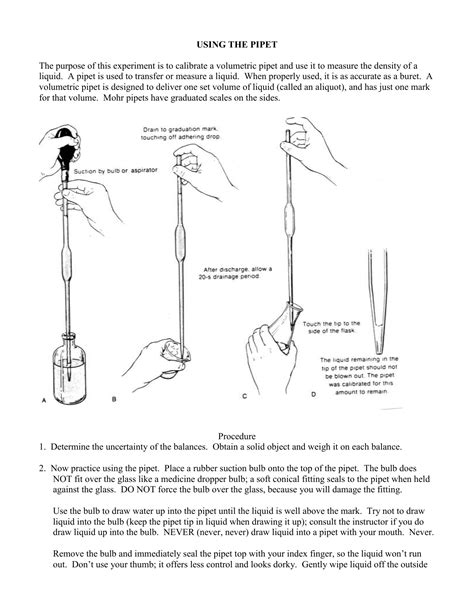
This step prevents contamination or dilution of the solution from water or other chemicals on the glassware’s inside walls and removes the need to dry the pipet. . A volumetric pipet is an elongated glass bulb with two narrow glass stems at the top and bottom of the bulb (Figure 2). The pipet is used "to deliver" a single, fixed volume of .Lab Technique 5: Using a Volumetric Pipet 3 Fill the Pipet: Depress the pipet bulb or valve A on the three-way pipet bulb to remove air as before. Release the pressure on the pipet bulb to suction liquid into the pipet until the meniscus is above the calibration line (squeeze “S” on the three-way pipet bulb). The most precise volume measurements are done with pipets, burets, and volumetric flasks. Cleaning Glassware. Good volumetric technique requires clean glassware. The standard test for cleanliness of volumetric flasks, pipets and burets is to fill them with liquid and then let them drain. If droplets of liquid cling to the inner walls, the glass . We also can accomplish the same dilution in two steps using a 50-mL pipet and 100-mL volumetric flask for the first dilution, and a 10-mL pipet and a 50-mL volumetric flask for the second dilution. The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the volumetric .
volumetric pipette solution
Pipette Calibration Calculation. The density of water as a function of temperature is given in the second column of Table \(\PageIndex{1}\). Use the density for your temperature to calculate the volume of water delivered by your pipette in each determination, the mean volume, the standard deviation, and the 50% and 95% confidence limits for the mean.The solution has been diluted by one-fifth since the new volume is five times as great as the original volume. Consequently, the molarity is one-fifth of its original value. Another common dilution problem involves calculating what amount of a highly concentrated solution is required to make a desired quantity of solution of lesser concentration.dissolution of a known mass of solid or the by dilution of a more concentrated solution x A volumetric pipette is used to deliver a single fixed-volume of liquid, at a specific temperature, . reaches the volumetric mark. Touch the tip of the pipette to the side of the container to ensure that no drop is present at the tip of the pipette .
250 mL of the stock HNO 3 needs to be diluted with water to a final volume of 8.00 L. The dilution is by a factor of 32 to go from 16 M to 0.5 M. Image 1. Volumetric pipette. Image from . a glass pipette is being used to transfer a portion of a solution from a cylinder.Volumetric pipette.Use of a pipette rather than a graduated cylinder for . About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright .how to use standard volumetric glassware to dilute a concentrated stock solution in order to prepare a more dilute solution in just such a manner. You will need: • a volumetric transfer pipette • a suitably sized volumetric flask • a stock solution of known concentration • a supply of solvent, normally distilled or deionised water 50 mL volumetric flask ; 50 mL beaker ; 10 mL graduated cylinder ; From the 250 mL beaker, transfer a little less than the volume of water listed for the glassware below, one piece of glassware at a time. Use a plastic disposable pipet to add the water drop-wise until the bottom of the meniscus is on the line that corresponds to the desired volume.
To calculate a dilution, you use the formula: C1 * V1 = C2 * V2 . Example: To perform a reaction, you need to dilute your stock solution in water. . The pipette name is the largest volume it can measure with the letter ‘P’ added. Common pipette sizes and their volume ranges include: P10: 0.5 μL – 10 μL P20: 2 μL – 20 μL P100: 10 .very accurate total volume. Note that unlike volumetric pipettes, volumetric flasks do not account for liquid that remains after you pour out the contents. If you need to measure out 10.00 mL of a solution for a reaction, you should use a 10.00 mL pipette instead of simply pouring out the contents of a 10.00 mL volumetric flask. Other Equipmentdissolution of a known mass of solid or the by dilution of a more concentrated solution x A volumetric pipette is used to deliver a single fixed-volume of liquid, at a specific temperature, . reaches the volumetric mark. Touch the tip of the pipette to the side of the container to ensure that no drop is present at the tip of the pipette .
Example: Make only 300 μL of a 1:1000 dilution, assuming the smallest volume you can pipette is 2 μL; Choose step DFs: With a total dilution factor of 1000, you can do a 1:10 followed by a 1:100 (10 * 100 = 1000) . Formula for 1:100 Dilution: Final Volume / . Consider the number of combinations of standard glassware to make a single dilution. Assume that in the laboratory, there are pipettes from 1 mL to 25 mL and volumetric flasks from 5 mL to 1000 mL. Table II illustrates single step whole number dilution ratios from 5 to 1000. The dilution ratio here is 1/C of Equation 2.
Abstract. This guide is the second chapter in the Biotechnology 101 Kit, that teaches you the basics of hands-on molecular biology.The pipette is an indispensable tool when working with DNA and analysing genes, so you will need to be able to pipette with skill and confidence, before starting the research projects in this kit.
Since V 1 is the only variable that cannot be assigned to a numerical value in the given problem, the initial volume of the solution is the unknown quantity that will be calculated upon solving the dilution equation. The problem does not specify whether the final answer should be expressed in liters or milliliters. Therefore, the given unit for the final volume, "liters," is .The volumetric pipette is also called a volumetric pipette, since it presents gauges or measurements that establish the amount of liquid contained and its use is recommended when accuracy and reproducibility are crucial. They can be classified according to their degree of precision, volumetric pipettes of class A being the highest quality .Step 3: Calculate the moles of Na 2 CO 3 in the pipette which is transferred to the second volumetric flask for dilution c stock calculated above = 0.0943 mol L-1 Assume temperature of laboratory is the same as that required by the pipette (25 o C for example) V pipette = 50.00 mL convert volume of pipette in mL to volume in L
The volumetric pipette used in this lab is designed to measure and transfer exactly 5.00 mL of solution. First, rinse the inside of the volumetric pipette with distilled water. Using the pipette bulb, draw the water into the pipette up above the 5-mL mark, then allow it to drain out through the tip. You may want to do this several times for .the possible target results. Proper dilution of standards and samples is based on the understanding of basic dilution, volumetric procedures and dilution factors. Manufacturer, Country, Certifications Nominal Value Tolerance EX/IN To Deliver & To Contain Precision Class • Class A - Highest Quality • Class B - Qualitative Etchings, GraduationsMaking a single dilution In analytical work, you may need to dilute a standard solution to give a particular mass concentration or molar concentration. . Use [C 1] V 1 = [C 2]V 2 to calculate the volume of standard solution for each member of the series and pipette or syringe the calculated volume into an appropriately sized volumetric flask .ANALYTICAL SCIENCES DECEMBER 1994, VOL. 10 883 and independent of the dilution degree or the volume ratio of pipette to flask. Table 4 shows the results of the pipette-pipette dilu-
One of the more common applications being used in labs today, is the serial dilution. And INTEGRA electronic pipettes offer a mode, which will help you carry out all pipetting steps required to perform a dilution. . enter the serial dilution program from the main menu. Here we set the volume of the sample, which is to be diluted across the .Pipettes. Pipettes are used to measure out fixed volumes of liquids very accurately. The most common sizes are 25 cm 3 and 10 cm 3.Although pipettes come in other sizes, you are probably unlikely to come across a bigger one in an A level lab, although you might occasionally use a smaller one such as 5 cm 3.Small graduated pipettes are also available - rather like a dropper . A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step.. In serial dilutions, you multiply the dilution factors for each step. The dilution factor or the dilution is the initial volume divided by the final volume.. #DF = V_i/V_f# For example, if you add a 1 mL sample to 9 mL of diluent to get 10 mL of solution,
volumetric pipette chemistry
volumetric pipet procedure
volumetric pipet practice
is the pellet b test hard
The global autoclaved aerated concrete (AAC) market was valued at $4,498.5 million in 2019, and is expected to witness a CAGR of 6.0% during the forecast period. Increasing industrialization and urbanization has boosted the demand .The Autoclaved Aerated Concrete (AAC) market is experiencing significant growth, driven by the increasing demand for lightweight, environmentally friendly, and energy-efficient construction materials.
is volumetric pipette dilution|volumetric pipet procedure